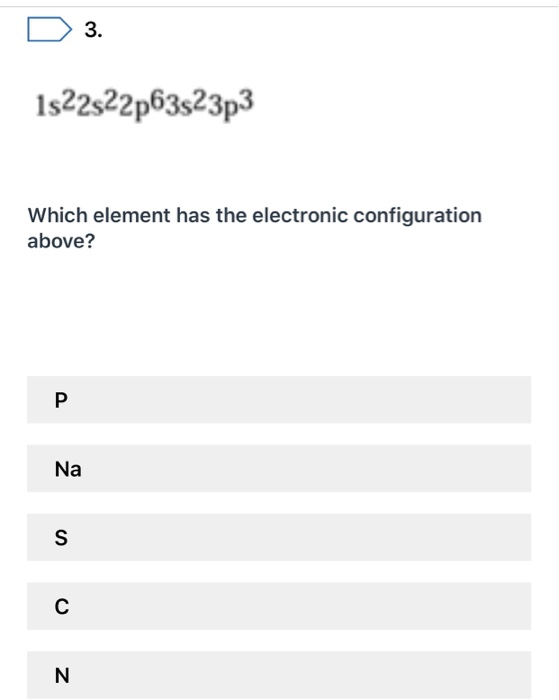
What Element Has the Electron Configuration 1s22s22p63s23p3
Step 2 of 5. Step 1 of 5.

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p3 Youtube
An atom that has the following electron configuration.

. Correct option is A. Check all possible answers. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Chapter 11 Problem 39QEP is solved. View a sample solution. Electronic configuration of chemical elements is the classification and ordering of electrons around the nucleus of an atom in reference to their energy levels.
Electrons can be ordered and classified using Aufbaus principle. To figure this out the element with the electron config of we first count the electrons. The electron configuration shows the distribution of electrons into subshells.
The element argon atomic number 18 has the electron configuration 1s22s22p63s23p6 which can be abbreviated Ne 3s23p6 What is the electron configuration for Ca2. To save room the configurations are in noble gas shorthandThis means part of the electron configuration has been replaced with the element symbol of the noble gas symbol. In which period and group of the periodic table is it located.
Which element has the electron configuration 1s22s22p63s23p3. 2 The element with an electron configuration of 1s22s22p63s23p3 is in group and period. What is the electronic configuration for the element phosphorus.
Therefore the Chlorine electron configuration will be 1s22s22p63s23p5. A positron is a particle emitted from the nucleus that has the same mass as an electron but has a positive charge The damaging effects of radiation on the body are a result of. Contains four electrons in its third and outer main energy level d.
1s22s22p63s23p3 contains only 5 valence electrons in its outermost shell. 100 4 ratings for this solution. 4 rows Well put six in the 2p orbital and then put the next two electrons in the 3s.
What is the atomic number of the element which is just below the above-given element in the periodic table. How many unpaired electrons are in an atom of chromium Cr in its ground-state. 2 The element with an electron configuration of 1s22s22p63s23p3 is in group and period.
The electronic configuration of an element is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3. Element with electron configuration 1s2 2s2 2p3 is Nitrogen N It has two electrons in 1s two in 2s and three in 2p arbitrarily two in 2px and 1 in 2py 2. All the atoms are 18 2 10 3 33.
What element has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 4. Which ion has the ground-state electron configuration. The element with an electronic configuration of Ar4s23d104p3 A r 4 s 2 3 d 10 4 p 3 is Arsenic.
Phosphorus has electron configuration 1s22s22p63s23p3. What is the electron configuration for the Potassium K atom in its ground-state. Which element has the electron configuration 1s22s22p63s23p3.
View this answer View this answer View this answer done loading. See the answer See. If an element has atoms in their.
The element with atomic number 33 in the periodic table is Arsenic. This is because Ar represents 18 atoms 2 atoms are in 4s2 4 s 2 a0 atoms in 3d10 and 3 atoms in 4p3. Contains one set of paired and three unpaired electrons in its fourth and outer main energy level.
Identify the elements have the following electron configurations. The element is phosphorus and it belongs to group 15 period 3. Those are the small number placed at the top after the letters.
This list of electron configurations of elements contains all the elements in increasing order of atomic number. Since the 3s if now. Which of the following elements would exhibit the greatest shielding effect.
Solved 3 1s22s22p63s23p3 Which Element Has The Electronic Chegg Com

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p3 Youtube

Solved Give The Symbol Of The Atom With The Electron Chegg Com
No comments for "What Element Has the Electron Configuration 1s22s22p63s23p3"
Post a Comment